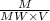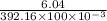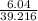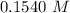Chemistry, 26.05.2021 22:50 veronicacalyn
A student weighs out a 6.04 g sample of , transfers it to a 100. mL volumetric flask, adds enough water to dissolve it and then adds water to the 100. mL tick mark. What is the molarity of chromium(III) sulfate in the resulting solution

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
A student weighs out a 6.04 g sample of , transfers it to a 100. mL volumetric flask, adds enough wa...
Questions

Mathematics, 27.10.2020 04:40

Geography, 27.10.2020 04:40

Mathematics, 27.10.2020 04:40

Mathematics, 27.10.2020 04:40

Biology, 27.10.2020 04:40




English, 27.10.2020 04:40

Mathematics, 27.10.2020 04:40




English, 27.10.2020 04:40

Mathematics, 27.10.2020 04:40

Mathematics, 27.10.2020 04:40