
Chemistry, 27.05.2021 14:00 mikaelalcool1
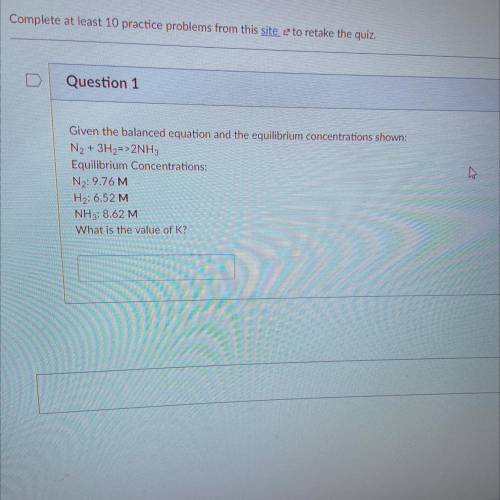
Given the balanced equation and the equilibrium concentrations shown:
N2 + 3H2=>2NH3
Equilibrium Concentrations:
N2: 9.76 M
H2: 6.52 M
NH3: 8.62 M
What is the value of K?

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

You know the right answer?
Given the balanced equation and the equilibrium concentrations shown:
N2 + 3H2=>2NH3
Equil...
Equil...
Questions

Biology, 05.09.2020 01:01

Chemistry, 05.09.2020 01:01

History, 05.09.2020 01:01


Mathematics, 05.09.2020 01:01

Social Studies, 05.09.2020 01:01


Law, 05.09.2020 01:01






English, 05.09.2020 01:01




Social Studies, 05.09.2020 01:01


Business, 05.09.2020 01:01

![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/1350/9607/eda24.png)
![Kc=\frac{[NH_{3} ]^{2} }{[N_{2} ]*[H_{2} ]^{3} }](/tpl/images/1350/9607/9d540.png)



