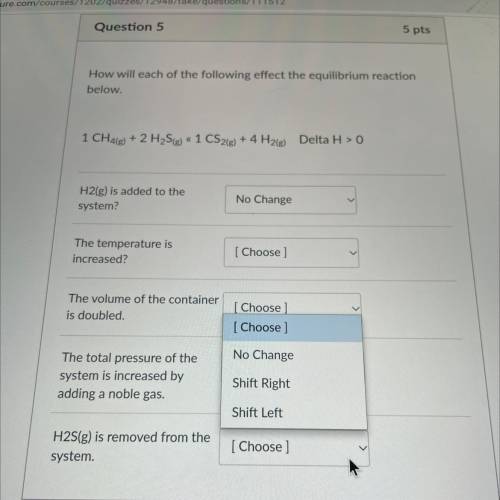
How will each of the following effect the equilibrium reaction
below.
1 CH4(g) + 2 H2S(g) « 1...

Chemistry, 27.05.2021 21:10 gerardoblk5931
How will each of the following effect the equilibrium reaction
below.
1 CH4(g) + 2 H2S(g) « 1 CS2(g) + 4 H2(g) Delta H > 0
HELPOPO

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Questions





Chemistry, 20.07.2019 19:30


Chemistry, 20.07.2019 19:30

Computers and Technology, 20.07.2019 19:30

Computers and Technology, 20.07.2019 19:30

Computers and Technology, 20.07.2019 19:30

Computers and Technology, 20.07.2019 19:30



Computers and Technology, 20.07.2019 19:30




Social Studies, 20.07.2019 19:30

Mathematics, 20.07.2019 19:30



