Chemistry, 27.05.2021 22:40 microwave13016
1. For each of the following formulas:
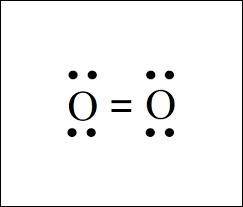
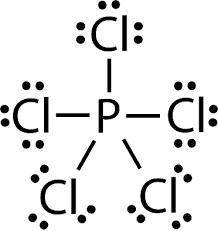
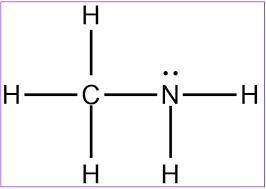
1) if ionic, write the formulas of the ions; if covalent, draw the Lewis structure
2) For each covalent compound, describe the electronic and molecular geometry
3) For each covalent compound, describe the hybridization of the central atom
4) Name each compound, except the organic one.
5) How many sigma and how many pi bonds does each compound have?
MnSO4 CH3NH2 PCl5 O2 LiF


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

You know the right answer?
1. For each of the following formulas:
1) if ionic, write the formulas of the ions; if covalent, dr...
Questions




Mathematics, 29.01.2021 19:20

Mathematics, 29.01.2021 19:20




Mathematics, 29.01.2021 19:20

Computers and Technology, 29.01.2021 19:20



English, 29.01.2021 19:20

Mathematics, 29.01.2021 19:20

Law, 29.01.2021 19:20

Mathematics, 29.01.2021 19:20

History, 29.01.2021 19:20


English, 29.01.2021 19:20

Mathematics, 29.01.2021 19:20