How much energy is released when 6.0 g of water is condensed from water
vapor?
A. 6.0 g x 1 m...

Chemistry, 28.05.2021 01:00 malachitorres813
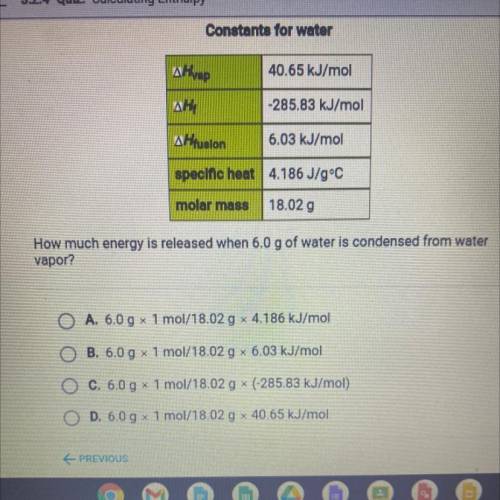
How much energy is released when 6.0 g of water is condensed from water
vapor?
A. 6.0 g x 1 mol/18.02 g x 4.186 kJ/mol
B. 6.0 g 1 mol/18.02 g 6.03 kJ/mol
O C. 6.0 g x 1 mol/18.02 g * (-285.83 kJ/mol)
O D. 6.0 g x 1 mol/18.02 g x 40.65 kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Questions


Mathematics, 06.04.2021 17:00


Mathematics, 06.04.2021 17:00


Mathematics, 06.04.2021 17:00

History, 06.04.2021 17:00

Chemistry, 06.04.2021 17:00

Mathematics, 06.04.2021 17:00

Chemistry, 06.04.2021 17:00


Mathematics, 06.04.2021 17:00








History, 06.04.2021 17:00



