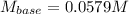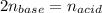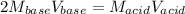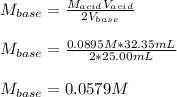Chemistry, 28.05.2021 01:10 dbzrules02
It takes 32.35 mL of a 0.0895 M hydrochloric acid solution to reach the equivalence point in the reaction with 25.00 mL of barium hydroxide. 2HCl(aq) Ba(OH)2(aq) 2H2O(l) BaCl2(aq) What is the molar concentration of the barium hydroxide solution

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
It takes 32.35 mL of a 0.0895 M hydrochloric acid solution to reach the equivalence point in the rea...
Questions

History, 05.06.2021 18:50

Chemistry, 05.06.2021 18:50


Mathematics, 05.06.2021 19:00

History, 05.06.2021 19:00



Mathematics, 05.06.2021 19:00

Mathematics, 05.06.2021 19:00



History, 05.06.2021 19:00

Business, 05.06.2021 19:00

Mathematics, 05.06.2021 19:00

World Languages, 05.06.2021 19:00



Mathematics, 05.06.2021 19:00


Mathematics, 05.06.2021 19:00