Chemistry, 28.05.2021 03:10 alissa2151
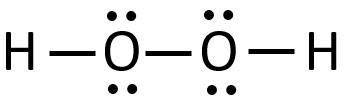
Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves a stable noble-gas electron configuration. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

You know the right answer?
Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves a stable noble-...
Questions




Mathematics, 13.11.2019 03:31



Physics, 13.11.2019 03:31

Mathematics, 13.11.2019 03:31

English, 13.11.2019 03:31

History, 13.11.2019 03:31

Business, 13.11.2019 03:31

Mathematics, 13.11.2019 03:31

Physics, 13.11.2019 03:31


Geography, 13.11.2019 03:31

History, 13.11.2019 03:31