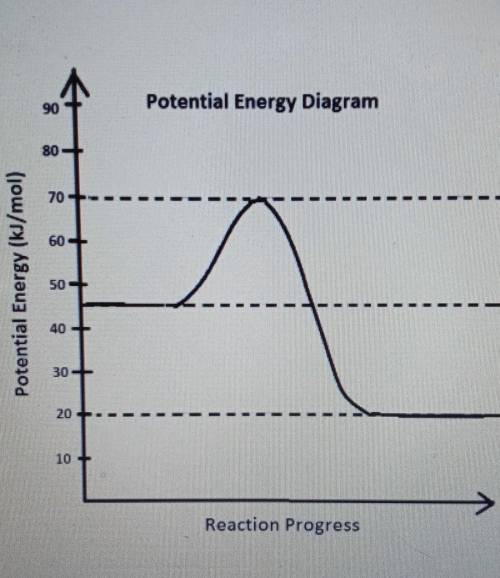
What is the the heat of reaction, delta, in kJ/mol?
a) -26kJ/mol
b) -25kJ/mol
c) -10kJ/mo...

Chemistry, 28.05.2021 05:40 Dmoney7784
What is the the heat of reaction, delta, in kJ/mol?
a) -26kJ/mol
b) -25kJ/mol
c) -10kJ/mol
d)-35kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Questions


History, 24.06.2019 15:00

Mathematics, 24.06.2019 15:00

Computers and Technology, 24.06.2019 15:00

Computers and Technology, 24.06.2019 15:00

Chemistry, 24.06.2019 15:00



History, 24.06.2019 15:00

Biology, 24.06.2019 15:00





Social Studies, 24.06.2019 15:00


Physics, 24.06.2019 15:00



Social Studies, 24.06.2019 15:00



