
Chemistry, 29.05.2021 01:40 Queenbee2304
Calculate the pH of a 0.10 M solution of acidic acid HC2H3O2(aq) at 25 °C. Ka for HC2H3O2 = 1.8×10−5 at 25 °C g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1


Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
Calculate the pH of a 0.10 M solution of acidic acid HC2H3O2(aq) at 25 °C. Ka for HC2H3O2 = 1.8×10−5...
Questions

Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00


Computers and Technology, 28.01.2021 17:00


Mathematics, 28.01.2021 17:00

Chemistry, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00


Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00


Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00

Mathematics, 28.01.2021 17:00


Mathematics, 28.01.2021 17:00

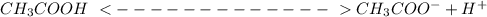
![Ka = \frac{[CH3COO^{-}] [H^{+}]}{[CH_3COOH]}\\\\](/tpl/images/1354/5896/b6175.png)
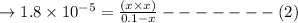
![1.8\times 10^{-5} = \frac{x^2}{0.10}\\\\x^2 = 1.8 \times 10^{-6}\\\\x = 1.34 \times 10^{-3}\\\\pH = -\log [H^{+}]](/tpl/images/1354/5896/b9098.png)
![[H^{+}] = x = 1.34 \times 10^{-3}](/tpl/images/1354/5896/38a1b.png)



