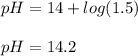Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
A chemist mixes 50.0mL of a 1.0M NaOH solution with 50.0mL of a 1.0M Ba(OH)2 solution. Assuming the...
Questions



Mathematics, 01.12.2020 19:50

Spanish, 01.12.2020 19:50




Mathematics, 01.12.2020 19:50

Social Studies, 01.12.2020 19:50



Mathematics, 01.12.2020 19:50




Physics, 01.12.2020 19:50

Mathematics, 01.12.2020 19:50


Chemistry, 01.12.2020 19:50

Mathematics, 01.12.2020 20:00

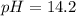
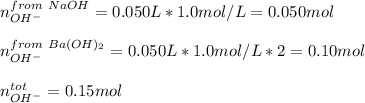
![[OH^-]=\frac{0.15mol}{0.100L}=1.5](/tpl/images/1354/5960/12bf6.png)