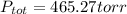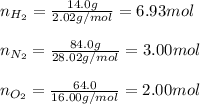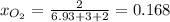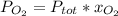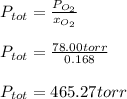Chemistry, 29.05.2021 02:50 kaliyab191
A mixture of 14.0 grams of H2, 84.0 grams of N2, and 64.0 grams of O2 are placed in a flask. The partial pressure of the O2 is 78.00 torr. What is the total pressure in the flask

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2

Chemistry, 23.06.2019 14:00
How is the electron sea model of metallic bonding different from the band theory? how are they the same? give at least one similarity and one difference between the models
Answers: 2

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1
You know the right answer?
A mixture of 14.0 grams of H2, 84.0 grams of N2, and 64.0 grams of O2 are placed in a flask. The par...
Questions

Computers and Technology, 05.10.2019 22:10

Social Studies, 05.10.2019 22:10





Chemistry, 05.10.2019 22:10


Physics, 05.10.2019 22:10


Mathematics, 05.10.2019 22:10

Mathematics, 05.10.2019 22:10

History, 05.10.2019 22:10

Mathematics, 05.10.2019 22:10

History, 05.10.2019 22:10

Mathematics, 05.10.2019 22:10

History, 05.10.2019 22:10

Mathematics, 05.10.2019 22:10


Geography, 05.10.2019 22:10