
Chemistry, 29.05.2021 03:30 mothertrucker2828
a compound decomposes by a first-order process. if 17.0% of the compound decomposes in 60.0 minutes, the half-life of the compound is

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
a compound decomposes by a first-order process. if 17.0% of the compound decomposes in 60.0 minutes,...
Questions


Advanced Placement (AP), 20.09.2020 17:01



Business, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

History, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Social Studies, 20.09.2020 17:01

Physics, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01





Mathematics, 20.09.2020 17:01

Geography, 20.09.2020 17:01


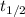 = 0.693 / k
= 0.693 / k

