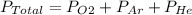Chemistry, 29.05.2021 04:30 bacchus2847
The total pressure of an O2-Ar-He gas mixture is 43 atm. If the partial pressure of Ar is 15 atm and the partial pressure of He is 7 atm, then the partial pressure of O2 is -

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
The total pressure of an O2-Ar-He gas mixture is 43 atm. If the partial pressure of Ar is 15 atm and...
Questions


Mathematics, 27.10.2021 07:20






Computers and Technology, 27.10.2021 07:20


Computers and Technology, 27.10.2021 07:20

English, 27.10.2021 07:20

English, 27.10.2021 07:20

Mathematics, 27.10.2021 07:20



Mathematics, 27.10.2021 07:20

Mathematics, 27.10.2021 07:20



Mathematics, 27.10.2021 07:20