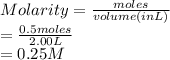Chemistry, 29.05.2021 09:40 dakshshberry
0.500 mile of potassium oxide is dissolved in enough water to make 2.00 L of solution. Calculate the molarity of this solution (plz help!) plz no links

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
0.500 mile of potassium oxide is dissolved in enough water to make 2.00 L of solution. Calculate the...
Questions

Advanced Placement (AP), 22.01.2021 01:20



English, 22.01.2021 01:20




History, 22.01.2021 01:20

Computers and Technology, 22.01.2021 01:20

Mathematics, 22.01.2021 01:20

Mathematics, 22.01.2021 01:20

History, 22.01.2021 01:20

Mathematics, 22.01.2021 01:20


Chemistry, 22.01.2021 01:20

Mathematics, 22.01.2021 01:20

Mathematics, 22.01.2021 01:20