
Chemistry, 30.05.2021 17:00 AtlFan6392
Given the reaction: N2 + O2 = 2NO for which the Keq at 2273 K is 1.2 x 10-4
a. Write the equilibrium constant expression for the reaction.
b. Write the equation that would allow you solve for the concentration of NO.
c. What is the concentration of NO if [NZ] = 0.166M and [02] = 0.145M?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Given the reaction: N2 + O2 = 2NO for which the Keq at 2273 K is 1.2 x 10-4
a. Write the equilibriu...
Questions


Mathematics, 13.10.2020 14:01

Biology, 13.10.2020 14:01



History, 13.10.2020 14:01

History, 13.10.2020 14:01





English, 13.10.2020 14:01


Mathematics, 13.10.2020 14:01

Social Studies, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01


Mathematics, 13.10.2020 14:01

![K_{eq}=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/1355/6643/3d926.png)
![[NO]=\sqrt{K_{eq}\times [N_2]\times [O_2]}](/tpl/images/1355/6643/c62a1.png)
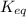
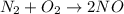
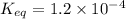
![[N_2]_{eq}=0.166M](/tpl/images/1355/6643/d7b28.png)
![[O_2]_{eq}=0.145M](/tpl/images/1355/6643/728fd.png)
![[NO]=\sqrt{(1.2\times 10^{-4})\times 0.166\times 0.145}](/tpl/images/1355/6643/e15e8.png)
![[NO]=\sqrt{2.88\times 10^{-6}}](/tpl/images/1355/6643/b8658.png)
![[NO]=0.0017 M](/tpl/images/1355/6643/d220d.png)


