

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Given the following reaction:
2 ZnS(s) + 3 O2(g) → 2 ZnO(s) + 2 SO2(g) ΔH = -927.54 kJ
...
...
Questions

Mathematics, 11.02.2020 11:20


English, 11.02.2020 11:22

Mathematics, 11.02.2020 11:34

English, 11.02.2020 11:34



English, 11.02.2020 11:36


English, 11.02.2020 11:37

History, 11.02.2020 11:37

World Languages, 11.02.2020 11:37

History, 11.02.2020 11:37


Physics, 11.02.2020 11:39


Social Studies, 11.02.2020 11:39

English, 11.02.2020 11:55

Mathematics, 11.02.2020 11:55

Mathematics, 11.02.2020 11:55

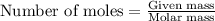 ......(1)
......(1)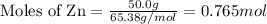
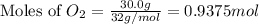

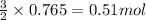 of oxygen gas
of oxygen gas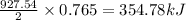 of energy
of energy

