
Chemistry, 31.05.2021 06:00 Mintfu2580
How many moles of Fe(OH), are produced when 85.0 L of iron(III)
sulfate at a concentration of 0.600 mol/L reacts with excess
NaOH?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
How many moles of Fe(OH), are produced when 85.0 L of iron(III)
sulfate at a concentration of 0.600...
Questions



Mathematics, 16.04.2021 21:40




Chemistry, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40



Mathematics, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

Chemistry, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40


Biology, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

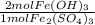 = 102 mol Fe(OH)₃
= 102 mol Fe(OH)₃

