Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1



Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 1
You know the right answer?
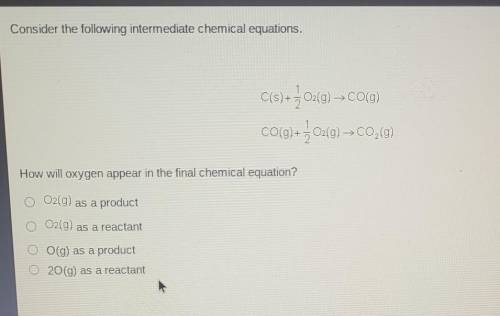
Consider the following intermediate chemical equations.
C(s) + + O2(g) → CO(g) CO(g) + } 02(g) C0,0...
Questions

Geography, 29.08.2019 16:30

English, 29.08.2019 16:30

History, 29.08.2019 16:30

Mathematics, 29.08.2019 16:30

Mathematics, 29.08.2019 16:30




Geography, 29.08.2019 16:30

History, 29.08.2019 16:30

Biology, 29.08.2019 16:30


English, 29.08.2019 16:30

Physics, 29.08.2019 16:30

History, 29.08.2019 16:30



Chemistry, 29.08.2019 16:30