
Chemistry, 01.06.2021 01:00 ethan62211
From the data presented in the report sheet, you can deduce that the stoichiometric coefficient for oxygen is . H20 (1) --> H2 (g) + O2 (g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1


Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
From the data presented in the report sheet, you can deduce that the stoichiometric coefficient for...
Questions

Mathematics, 25.05.2021 04:40


Mathematics, 25.05.2021 04:40



Social Studies, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40



Mathematics, 25.05.2021 04:40

Computers and Technology, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40



Chemistry, 25.05.2021 04:40



Social Studies, 25.05.2021 04:40

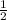 .
.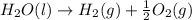
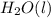 is 1, for
is 1, for 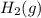 is 1 and for
is 1 and for 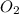 is
is 


