
Chemistry, 01.06.2021 01:20 asdf334asdf334
In the acetylene torch, acetylene gas (C2H2) burns in oxygen to produce carbon dioxide water and energy. How many moles of CO2 are formed from the reaction with 1.20 moles of C2H2?
Given the following equation
2C2H2(g) + 502(g) = 4CO2(g) + 2H2O(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
In the acetylene torch, acetylene gas (C2H2) burns in oxygen to produce carbon dioxide water and ene...
Questions


English, 28.09.2020 14:01

Social Studies, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Chemistry, 28.09.2020 14:01





English, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01



Arts, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01

Geography, 28.09.2020 14:01


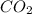 will be formed in the reaction.
will be formed in the reaction.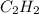 = 1.20 moles
= 1.20 moles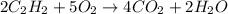
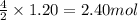 of carbon dioxide
of carbon dioxide

