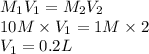A 10 M concentrated stock solution of NaCl is used to prepare 2 liters of diluted 1 M
solution. Which of the following is true for the process used to achieve the required
dilution? (5 points)
O The volume of the solvent used is less than 0.1 liters.
O The volume of stock solution used is less than 0.1 liters.
The volume of stock solution used is more than 2 liters.
The volume of the solvent used is less than 2 liters.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
A 10 M concentrated stock solution of NaCl is used to prepare 2 liters of diluted 1 M
solution. Whi...
Questions


Mathematics, 31.05.2021 18:20





Mathematics, 31.05.2021 18:20


English, 31.05.2021 18:30



Mathematics, 31.05.2021 18:30


Mathematics, 31.05.2021 18:30





Mathematics, 31.05.2021 18:30

English, 31.05.2021 18:30

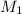 = 10 M,
= 10 M, 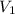 = ?
= ?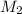 = 1 M,
= 1 M, 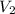 = 2 L
= 2 L