Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2
You know the right answer?
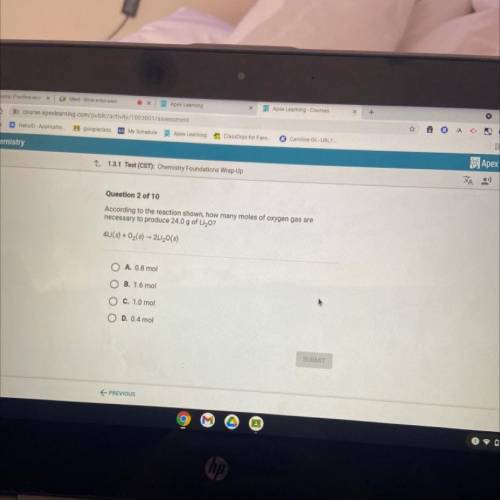
According to the reaction shown, how many moles of oxygen gas are
necessary to produce 24.0 g of Li...
Questions



Mathematics, 27.10.2019 02:43




Chemistry, 27.10.2019 02:43



History, 27.10.2019 02:43

Social Studies, 27.10.2019 02:43

History, 27.10.2019 02:43

Biology, 27.10.2019 02:43

Spanish, 27.10.2019 02:43



Biology, 27.10.2019 02:43

Geography, 27.10.2019 02:43

Mathematics, 27.10.2019 02:43