
Chemistry, 01.06.2021 20:00 twistedhyperboles
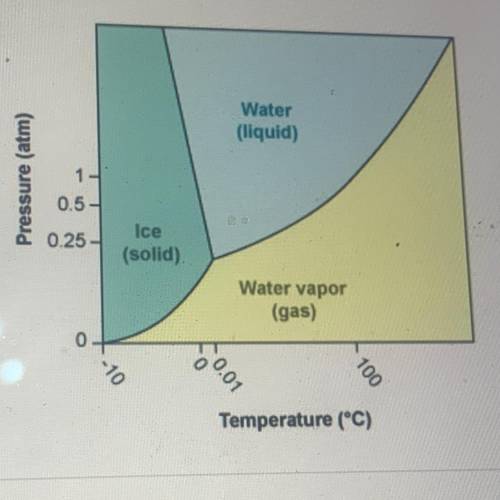
According to the phase diagram for H20, what happens to the phases of
water at 0°C as the pressure is increased from 0 atm to 10 atm?
A. Water changes from a gas to a solid to a liquid
B. Water changes from a liquid to a gas to a solid
C. Water changes from a liquid to a solid to a gas
D. Water changes from a solid to a liquid to a gas

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0°C as the pressure...
Questions

Chemistry, 04.09.2020 16:01





Biology, 04.09.2020 16:01

Mathematics, 04.09.2020 16:01

Mathematics, 04.09.2020 16:01

Chemistry, 04.09.2020 16:01

Physics, 04.09.2020 16:01

Mathematics, 04.09.2020 16:01


Mathematics, 04.09.2020 16:01

French, 04.09.2020 16:01


Chemistry, 04.09.2020 16:01

Chemistry, 04.09.2020 16:01


Mathematics, 04.09.2020 16:01

History, 04.09.2020 16:01



