Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 18:30
Calcium hydroxide and hydrochloric acid react to form calcium chloride and water as shown in the chemical reaction. if the chemicals are present in exactly the correct ratios to fully use all of the ingredients, how many moles of water would be formed from 5 moles of hcl? (oh)2 + ? +
Answers: 1
You know the right answer?
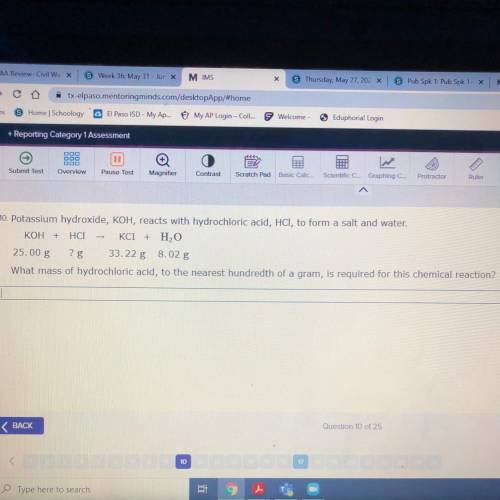
10. Potassium hydroxide, KOH, reacts with hydrochloric acid, HCl, to form a salt and water.
KOH + H...
Questions

English, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

Biology, 18.03.2021 01:30

English, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30




Chemistry, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30