
Chemistry, 02.06.2021 02:10 leeshaaa17
How many moles of oxygen would be needed to react with 6.78 moles of iron? PLEEASE SHOW WORK I WILL GIVE BRAINLIEST
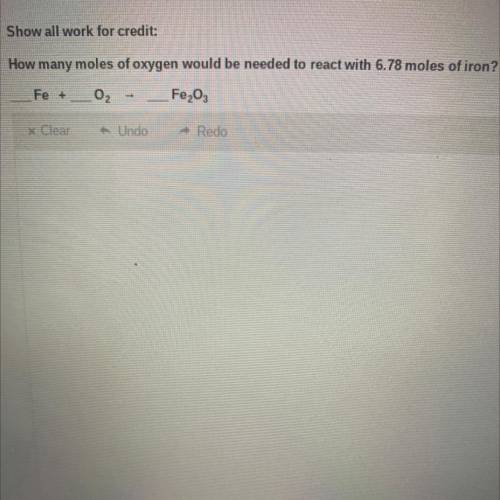

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2
You know the right answer?
How many moles of oxygen would be needed to react with 6.78 moles of iron?
PLEEASE SHOW WORK I WILL...
Questions


Mathematics, 13.02.2021 23:10

Computers and Technology, 13.02.2021 23:10









Mathematics, 13.02.2021 23:10







Business, 13.02.2021 23:10

English, 13.02.2021 23:10



