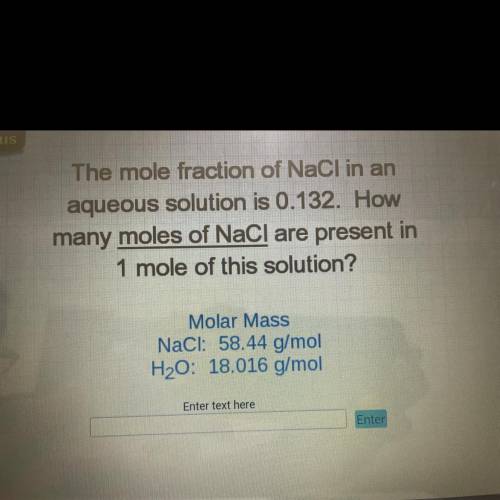
The mole fraction of NaCl in an
aqueous solution is 0.132. How
many moles of NaCl are present...


Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 21:50
The great plains are also referred to a. marshes c. interior plains b. lowlands d. plateaus select the best answer from the choices provided a b c d
Answers: 3
You know the right answer?
Questions



Mathematics, 06.05.2021 06:20



Mathematics, 06.05.2021 06:20


Mathematics, 06.05.2021 06:20

Spanish, 06.05.2021 06:20

Mathematics, 06.05.2021 06:20

Mathematics, 06.05.2021 06:20

Mathematics, 06.05.2021 06:20




Mathematics, 06.05.2021 06:20


English, 06.05.2021 06:20

English, 06.05.2021 06:20

Health, 06.05.2021 06:20