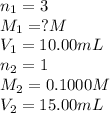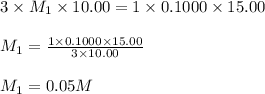Chemistry, 03.06.2021 04:30 elissiashontelbrown
g 10.00 mL of phosphoric acid (H3PO4) are titrated with 0.1000 M sodium hydroxide. 15.00 mL of the sodium hydroxide solution are used in this experiment. Determine the molarity of phosphoric acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
g 10.00 mL of phosphoric acid (H3PO4) are titrated with 0.1000 M sodium hydroxide. 15.00 mL of the s...
Questions

English, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30

Chemistry, 21.10.2019 21:30


Mathematics, 21.10.2019 21:30

Computers and Technology, 21.10.2019 21:30



English, 21.10.2019 21:30


Mathematics, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30


Mathematics, 21.10.2019 21:30

Biology, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30



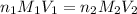 ........(1)
........(1)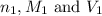 are the n-factor, molarity and volume of acid that is
are the n-factor, molarity and volume of acid that is 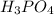
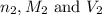 are the n-factor, molarity and volume of the base that is NaOH
are the n-factor, molarity and volume of the base that is NaOH