B. Hot water
The specific heat capacity of water is 4.186J/g °C
Calculate the temperature cha...

Chemistry, 03.06.2021 14:00 emilygoolsby2123
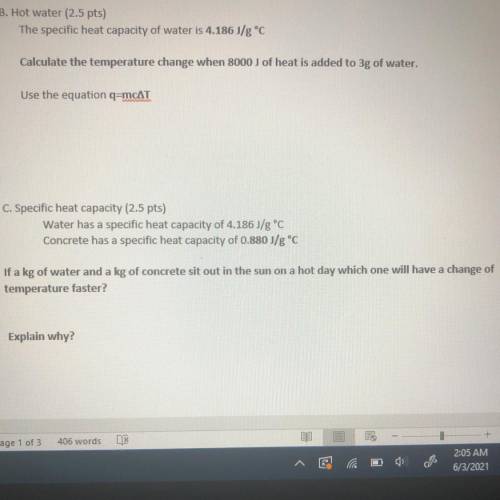
B. Hot water
The specific heat capacity of water is 4.186J/g °C
Calculate the temperature change when 8000 j of heat is added to 3g of water.
Use the equation q=mcAT
C. Specific heat capacity
Water has a specific heat capacity of 4.186J/g °C
Concrete has a specific heat capacity of 0.880 J/g °C
If a kg of water and a kg of concrete sit out in the sun on a hot day which one will have a change of temperature faster?
Explain why?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
Questions

Physics, 03.02.2020 21:54

History, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54

Mathematics, 03.02.2020 21:54





Mathematics, 03.02.2020 21:54


Mathematics, 03.02.2020 21:54

History, 03.02.2020 21:54



Social Studies, 03.02.2020 21:55


Mathematics, 03.02.2020 21:55

Mathematics, 03.02.2020 21:55



