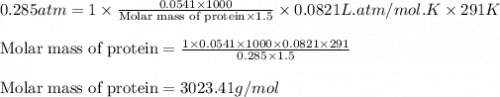Northern cod produce proteins that protect their cells from damage caused by subzero temperatures. Measurements of the osmotic pressure for two "antifreeze" proteins at 18°C yielded the data listed below. Use this information to calculate the molar mass for each of the proteins. Assume these proteins are nonelectrolytes and use the value i = 1.
Required:
If a 54.1 mg sample of protein A in 1.5 mL of water has an osmotic pressure of 0.285 atm, what is the molar mass of protein A?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1
You know the right answer?
Northern cod produce proteins that protect their cells from damage caused by subzero temperatures. M...
Questions

Mathematics, 19.03.2021 07:00

Mathematics, 19.03.2021 07:00


Mathematics, 19.03.2021 07:00




Mathematics, 19.03.2021 07:00

History, 19.03.2021 07:00

History, 19.03.2021 07:00


Mathematics, 19.03.2021 07:00

Mathematics, 19.03.2021 07:00


Social Studies, 19.03.2021 07:00

Mathematics, 19.03.2021 07:00

Mathematics, 19.03.2021 07:00



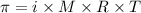
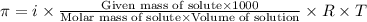 .....(1)
.....(1)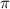 = osmotic pressure = 0.285 atm
= osmotic pressure = 0.285 atm![18^oC=[18+273]=291K](/tpl/images/1361/3277/7621e.png)