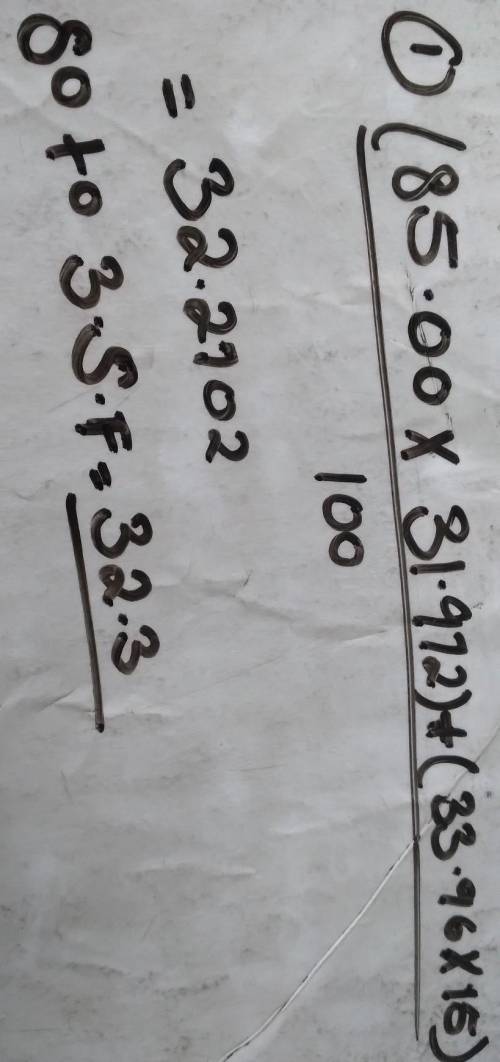Chemistry, 03.06.2021 21:20 birdman37361
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 amu (85.00 % abundance) and 33.96 amu (15.00% abundance)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 a...
Questions

Mathematics, 15.04.2020 23:51


History, 15.04.2020 23:51

Social Studies, 15.04.2020 23:51




Biology, 15.04.2020 23:51






Computers and Technology, 15.04.2020 23:52

History, 15.04.2020 23:52