

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
In the laboratory a student combines 47.8 mL of a 0.321 M aluminum nitrate solution with 21.8 mL of...
Questions

History, 02.09.2019 00:30

History, 02.09.2019 00:30

Mathematics, 02.09.2019 00:30






English, 02.09.2019 00:30


History, 02.09.2019 00:30



Geography, 02.09.2019 00:30

Chemistry, 02.09.2019 00:30




Mathematics, 02.09.2019 00:30

Mathematics, 02.09.2019 00:30

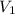 = 47.8 mL (1 mL = 0.001 L) = 0.0478 L
= 47.8 mL (1 mL = 0.001 L) = 0.0478 L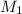 = 0.321 M,
= 0.321 M, 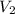 = 21.8 mL = 0.0218 L,
= 21.8 mL = 0.0218 L, 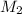 = 0.366 M
= 0.366 M![[Al^{3+}] = \frac{M_{1}V_{1} + M_{2}V_{2}}{V_{1} + V_{2}}](/tpl/images/1362/0108/5cce1.png)
![[Al^{3+}] = \frac{M_{1}V_{1} + M_{2}V_{2}}{V_{1} + V_{2}}\\= \frac{0.321 M \times 0.0478 L + 0.366 M \times 0.0218 L}{0.0478 L + 0.0218 L}\\= \frac{0.0153438 + 0.0079788}{0.0696}\\= 0.335 M](/tpl/images/1362/0108/11ccc.png)


