Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1


Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1
You know the right answer?
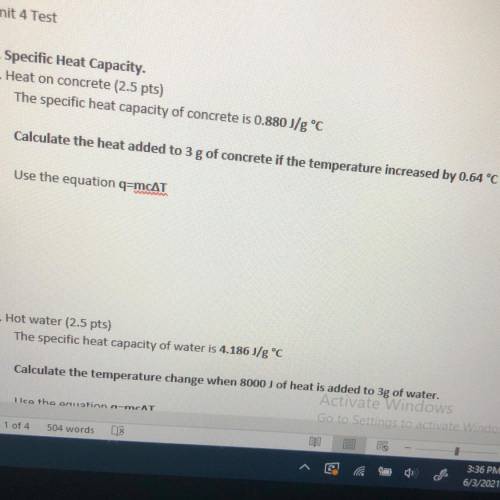
The specific heat capacity of water is 4.186 J/g °C
Calculate the temperature change when 8000 J of...
Questions

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00



Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00


Mathematics, 11.01.2021 07:00


Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00

Mathematics, 11.01.2021 07:00