
Chemistry, 04.06.2021 06:50 donnafranks2003
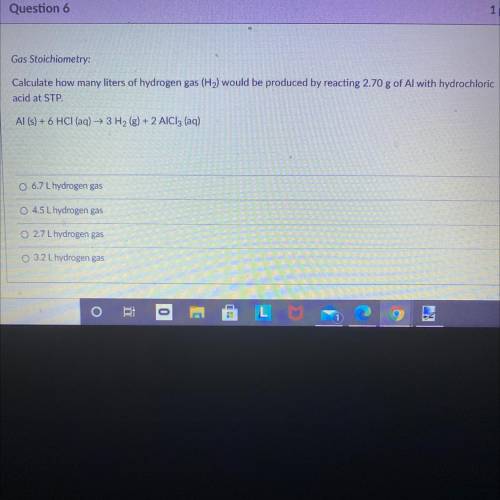
Gas Stoichiometry:
Calculate how many liters of hydrogen gas (H2) would be produced by reacting 2.70 g of Al with hydrochloric
acid at STP.
Al (s) + 6 HCl (aq) + 3H2(g) + 2 AlCl3 (aq)
6.7 L hydrogen gas
O 4.5 L hydrogen gas
O 2.7 L hydrogen gas
3.2 L hydrogen gas

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:40
Numbers with a end value of 4 or lower are rounded up, while with an end value of 5 or higher are rounded down. true or false?
Answers: 1

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
You know the right answer?
Gas Stoichiometry:
Calculate how many liters of hydrogen gas (H2) would be produced by reacting 2.7...
Questions








Mathematics, 22.10.2019 19:00






English, 22.10.2019 19:00


Business, 22.10.2019 19:00



Advanced Placement (AP), 22.10.2019 19:00



