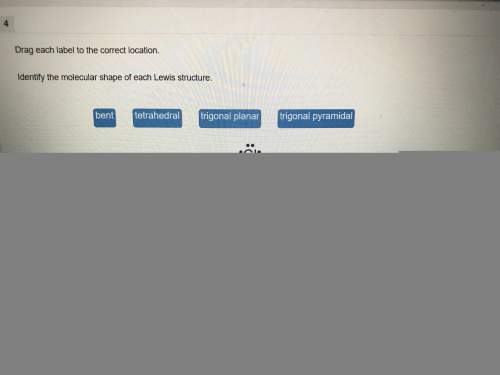
Chemistry, 04.06.2021 15:20 rleiphart1
The diagram shows the potential energy changes for a reaction pathway.
A curve line graph is shown. The y axis of the graph has the title Potential Energy. The x axis of the graph has the title Reaction Pathway. The curve begins at a lower level and ends at a slightly higher level. A vertical line labeled A, starts from the x axis till the beginning of the graph line. A vertical line labeled B starts from the x axis and continues till the peak of the graph. Another vertical line labeled C is shown from the x axis till the point where the curve ends.
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer.
Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway.
A curve line graph is shown....
Questions

English, 21.01.2020 23:31

English, 21.01.2020 23:31




English, 21.01.2020 23:31



Chemistry, 21.01.2020 23:31





Business, 21.01.2020 23:31


Mathematics, 21.01.2020 23:31

English, 21.01.2020 23:31