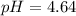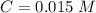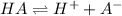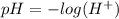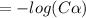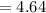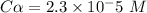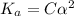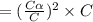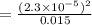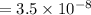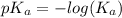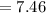Chemistry, 04.06.2021 18:20 FailingstudentXD
Calculate the pKa of hypochlorous acid (HClO, a weak acid). A 0.015 M solution of hypochlorous acid has a pH of 4.64.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
Calculate the pKa of hypochlorous acid (HClO, a weak acid). A 0.015 M solution of hypochlorous acid...
Questions


English, 20.09.2020 18:01


Biology, 20.09.2020 18:01




Mathematics, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01


Biology, 20.09.2020 18:01


Biology, 20.09.2020 18:01

Biology, 20.09.2020 18:01






Mathematics, 20.09.2020 18:01