
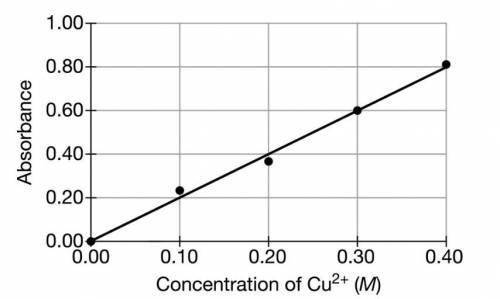
A student is given a sample of CuSO4(s) that contains a solid impurity that is soluble and colorless. The student wants to determine the amount of CuSO4 in the sample and decides to use a spectrophotometer. First, the student prepares a calibration graph by measuring the absorbances of CuSO4(aq) solutions of known concentrations. The graph is shown below.
(a) The student dissolves the entire impure sample of CuSO4(s) in enough distilled water to make 100.mL of solution. Then the student measures the absorbance of the solution and observes that it is 0.30. Determine the concentration of CuSO4(aq) in the solution.
(b) Calculate the number of moles of CuSO4 that were in the impure sample of CuSO4(s).
(c) In addition to the number of moles of CuSO4 calculated in part (b), what other quantity must be measured in order to calculate the mass percentage of CuSO4 in the impure sample of CuSO4(s)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
A student is given a sample of CuSO4(s) that contains a solid impurity that is soluble and colorless...
Questions

Mathematics, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

English, 13.07.2019 03:30

Biology, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Chemistry, 13.07.2019 03:30

Biology, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Computers and Technology, 13.07.2019 03:30


Biology, 13.07.2019 03:30

History, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30

English, 13.07.2019 03:30

Mathematics, 13.07.2019 03:30



