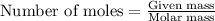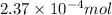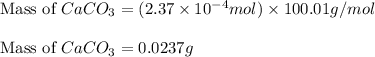Chemistry, 04.06.2021 20:20 jujulakaeuaws
Calcium carbonate is often used as an antacid. Your stomach acid is composed of HCl at a pH of 1.5. If you ate t much Turkey and need to neutralize 15.0 mL of stomach acid, how many grams of calcium carbonate would you need to take

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Calcium carbonate is often used as an antacid. Your stomach acid is composed of HCl at a pH of 1.5....
Questions

Physics, 23.10.2019 13:00





Computers and Technology, 23.10.2019 13:00



English, 23.10.2019 13:00


Chemistry, 23.10.2019 13:00

Health, 23.10.2019 13:00


Mathematics, 23.10.2019 13:00




Health, 23.10.2019 13:00

Mathematics, 23.10.2019 13:00

Biology, 23.10.2019 13:00

![pH=-\log [H^+]](/tpl/images/1363/1507/37e81.png) .....(1)
.....(1)![1.5=-\log[H^+]](/tpl/images/1363/1507/d7b07.png)
![[H^+]=10^{(-1.5)}=0.0316M](/tpl/images/1363/1507/dca45.png)
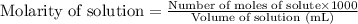 .....(2)
.....(2)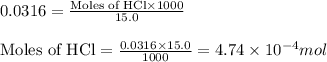
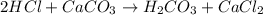
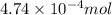 of HCl will react with =
of HCl will react with = 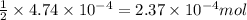 of calcium carbonate
of calcium carbonate