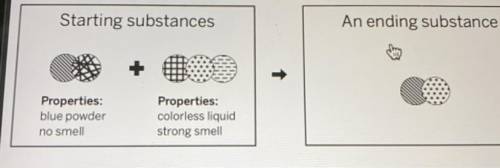
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a strong smell. Their repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same substance as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


You know the right answer?
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a s...
Questions













Computers and Technology, 25.08.2020 15:01



History, 25.08.2020 15:01

Computers and Technology, 25.08.2020 15:01



Computers and Technology, 25.08.2020 15:01