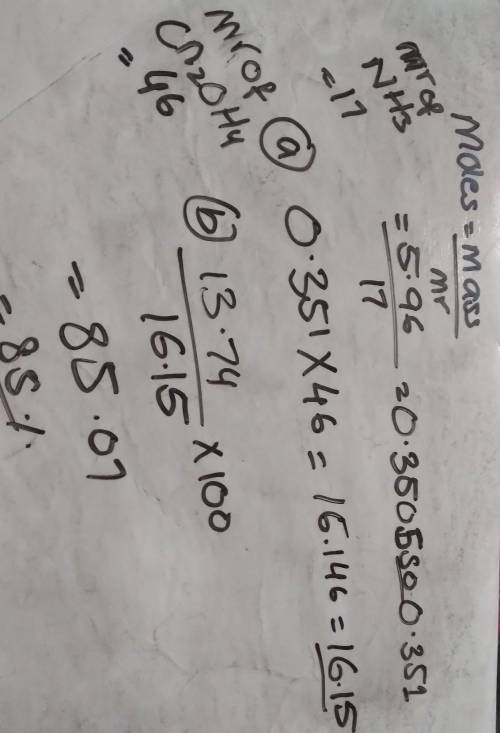Chemistry, 05.06.2021 22:40 GreenHerbz206
5.96 g of ammonia reacts completely according to the following reaction:
2 NH, (g) + Co, (g) → CN, OH, (s) + H20 (1)
(a) What is the theoretical yield of urea (CN, OH,) for this reaction?
(b) If 13.74 g of urea are produced, what is the percent yield for this equation?
please show work, will give brainliest

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1


Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 10:30
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
5.96 g of ammonia reacts completely according to the following reaction:
2 NH, (g) + Co, (g) → CN,...
Questions

Biology, 04.09.2020 17:01

Geography, 04.09.2020 17:01



Mathematics, 04.09.2020 17:01




Mathematics, 04.09.2020 17:01

Mathematics, 04.09.2020 17:01

French, 04.09.2020 17:01


Biology, 04.09.2020 17:01

History, 04.09.2020 17:01

English, 04.09.2020 17:01

Physics, 04.09.2020 17:01


History, 04.09.2020 17:01


Business, 04.09.2020 17:01