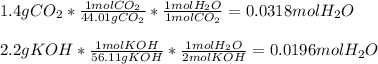Chemistry, 06.06.2021 01:10 Kaylenejohnson00
Identify the limiting reactant
1.4 g of CO2 and 2.2 g of KOH in the reaction: CO2 + 2KOH → K2CO3 + H20
Please show work, will give brainliest

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Identify the limiting reactant
1.4 g of CO2 and 2.2 g of KOH in the reaction: CO2 + 2KOH → K2CO3 +...
Questions

Mathematics, 15.10.2020 06:01

Mathematics, 15.10.2020 06:01


History, 15.10.2020 06:01

Mathematics, 15.10.2020 06:01


Chemistry, 15.10.2020 06:01





Mathematics, 15.10.2020 06:01

Computers and Technology, 15.10.2020 06:01


Mathematics, 15.10.2020 06:01


Mathematics, 15.10.2020 06:01


Arts, 15.10.2020 06:01

Geography, 15.10.2020 06:01