
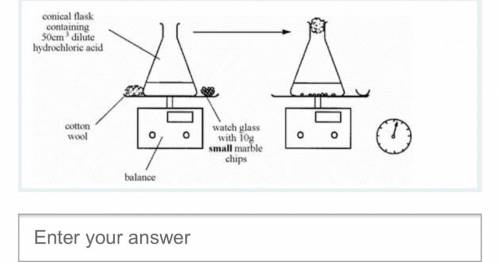
Marble chips (calcium carbonate) react with dilute hydrochloric acid.
calcium + hydrochloric → calcium + carbon + water
carbonate acid chloride dioxide
A student wanted to find out if the size of the marble chips made a difference to how fast the reaction took place. What two variables should she measure and take of?
PLEASE HELP I HAVE A TEST RIGHT NOW

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Marble chips (calcium carbonate) react with dilute hydrochloric acid.
calcium + hydrochloric → calc...
Questions

History, 21.08.2019 09:20

Mathematics, 21.08.2019 09:20


History, 21.08.2019 09:20

History, 21.08.2019 09:20

History, 21.08.2019 09:20



Mathematics, 21.08.2019 09:20


Mathematics, 21.08.2019 09:20

English, 21.08.2019 09:20

Mathematics, 21.08.2019 09:20




Social Studies, 21.08.2019 09:20

Mathematics, 21.08.2019 09:20





