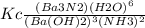The expression for the equilibrium constant for the reaction:
Ba3N2 (aq) + 6 H2O(1)-
3 Ba(OH)...

Chemistry, 07.06.2021 22:50 teddybear196510
The expression for the equilibrium constant for the reaction:
Ba3N2 (aq) + 6 H2O(1)-
3 Ba(OH)2 (aq) + 2 NH3 (9)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Questions


Mathematics, 10.11.2019 17:31

Biology, 10.11.2019 17:31


History, 10.11.2019 17:31

Mathematics, 10.11.2019 17:31

Mathematics, 10.11.2019 17:31




Mathematics, 10.11.2019 17:31


English, 10.11.2019 17:31

Mathematics, 10.11.2019 17:31




History, 10.11.2019 17:31


Arts, 10.11.2019 17:31