
Chemistry, 07.06.2021 22:50 naomicervero
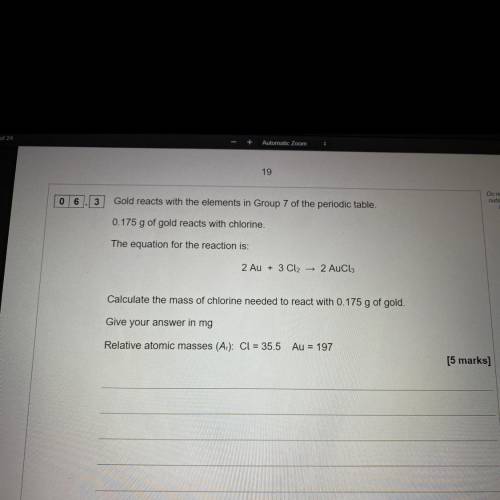
Gold reacts with elements in group 7 of the periodic table 0.175g of gold reacts with chlorine.
The equation for reaction for the reaction is
2Au + 3cl2 ——> 2Aucl3
Calculate the mass of chlorine needed to react with 0.175g of gold. Give your answer in mg
Relative atomic masses cl=35.5 Au=197

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1



Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
Gold reacts with elements in group 7 of the periodic table 0.175g of gold reacts with chlorine.
The...
Questions

History, 05.05.2020 18:09


Mathematics, 05.05.2020 18:09

Mathematics, 05.05.2020 18:09


Social Studies, 05.05.2020 18:09



Mathematics, 05.05.2020 18:09

Geography, 05.05.2020 18:09


Biology, 05.05.2020 18:09

Mathematics, 05.05.2020 18:09


Mathematics, 05.05.2020 18:09


Mathematics, 05.05.2020 18:09


Biology, 05.05.2020 18:09



