
Chemistry, 08.06.2021 08:50 elizabethprasad2
Assume that the reactions shown below are each an elementary step. What is molecularity for each reaction? What is rate law for each reaction?
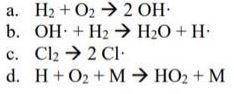

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Assume that the reactions shown below are each an elementary step. What is molecularity for each rea...
Questions




Mathematics, 20.11.2020 18:30



English, 20.11.2020 18:30


Mathematics, 20.11.2020 18:30

Mathematics, 20.11.2020 18:30


Health, 20.11.2020 18:30




Mathematics, 20.11.2020 18:40



Biology, 20.11.2020 18:40

Mathematics, 20.11.2020 18:40



