
Chemistry, 08.06.2021 16:40 AbigailHaylei
4Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
A. What is the oxidation number for each item in this equation?
B. What is being oxidized?
C. What is being reduced?
D. What is the oxidizing agent?
E. What is the reducing agent?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
4Fe (s) + 3 O2 (g) → 2 Fe2O3 (s)
A. What is the oxidation number for each item in this equation?
Questions


History, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00



Mathematics, 03.12.2020 01:00

Arts, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


History, 03.12.2020 01:00


English, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


English, 03.12.2020 01:00

Physics, 03.12.2020 01:00

English, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Physics, 03.12.2020 01:00

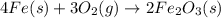
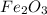 is +3.
is +3. 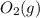 is 0 as it is present in its elemental state.
is 0 as it is present in its elemental state.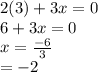
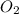 as a decrease in its oxidation state is occurring. So,
as a decrease in its oxidation state is occurring. So, 

