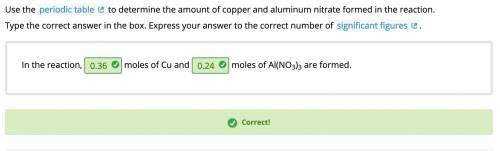
Use the periodic table to determine the amount of copper and aluminum nitrate formed in the reaction.
Type the correct answer in the box. Express your answer to the correct number of significant figures.
In the reaction,
moles of Cu and
moles of Al(NO3)3 are formed.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Use the periodic table to determine the amount of copper and aluminum nitrate formed in the reaction...
Questions

Mathematics, 31.08.2020 18:01


Mathematics, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01



Mathematics, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01


Mathematics, 31.08.2020 18:01


Mathematics, 31.08.2020 18:01

Chemistry, 31.08.2020 18:01


Chemistry, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01

Advanced Placement (AP), 31.08.2020 18:01

Mathematics, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01

Mathematics, 31.08.2020 18:01