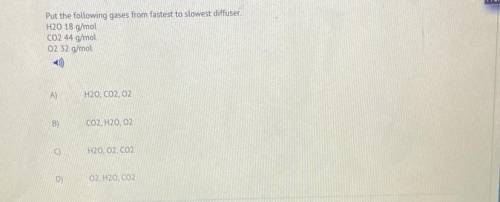
Put the following gases from fastest to slowest diffuser.
H20 18 g/mol
CO2 44 g/mol
02...

Chemistry, 08.06.2021 20:20 meowmeowcow
Put the following gases from fastest to slowest diffuser.
H20 18 g/mol
CO2 44 g/mol
02 32 g/mol
A)
H2O, CO2,02
B)
CO2, H20, 02
C)
H20, O2, CO2
D)
)
O2, H2O, CO2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Questions





Mathematics, 11.02.2020 18:59





History, 11.02.2020 18:59


Mathematics, 11.02.2020 19:00


Mathematics, 11.02.2020 19:00

History, 11.02.2020 19:00

Mathematics, 11.02.2020 19:00


Computers and Technology, 11.02.2020 19:01




