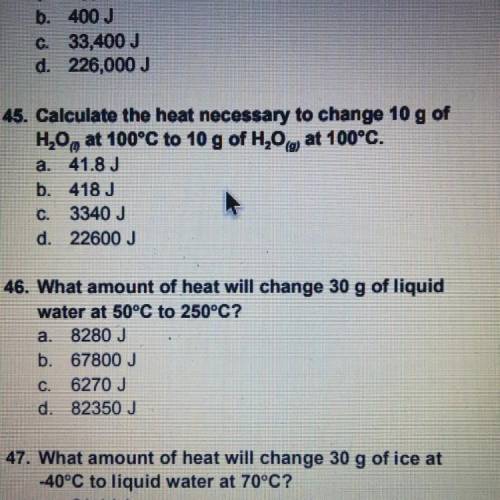Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
Calculate the heat necessary to change 10 g of H20, at 100°C to 10 g of H20 at 100°C.
a. 41.8 J
Questions

Business, 25.04.2021 16:00


English, 25.04.2021 16:00


Mathematics, 25.04.2021 16:00

Chemistry, 25.04.2021 16:00

Chemistry, 25.04.2021 16:00

Advanced Placement (AP), 25.04.2021 16:00

Mathematics, 25.04.2021 16:00

English, 25.04.2021 16:00

Health, 25.04.2021 16:10



Mathematics, 25.04.2021 16:10



Mathematics, 25.04.2021 16:10



Mathematics, 25.04.2021 16:10