

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
A certain liquid has a normal freezing point of and a freezing point depression constant . A solutio...
Questions




Mathematics, 28.11.2020 02:30


Biology, 28.11.2020 02:30



Mathematics, 28.11.2020 02:30









Mathematics, 28.11.2020 02:30


Health, 28.11.2020 02:30

 and a molal freezing point depression constant
and a molal freezing point depression constant  . A solution is prepared by dissolving some glycine in 950. g of X. This solution freezes at
. A solution is prepared by dissolving some glycine in 950. g of X. This solution freezes at  . Calculate the mass of urea that was dissolved. Round your answer to 2 significant digits.
. Calculate the mass of urea that was dissolved. Round your answer to 2 significant digits.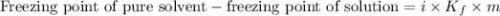
 ....(1)
....(1) = freezing point depression constant =
= freezing point depression constant = 
 = Molar mass of solute (glycine) = 75.07 g/mol
= Molar mass of solute (glycine) = 75.07 g/mol = Mass of solvent = 950 g
= Mass of solvent = 950 g


