Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
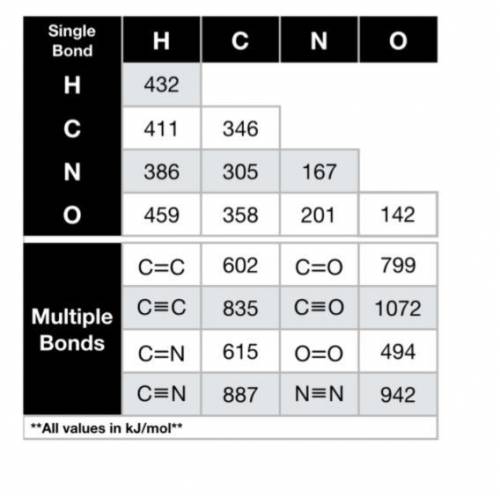
Using the bond energies provided, calculate the enthalpy of the reaction (∆Hrxn, in kJ) for the comb...
Questions

Social Studies, 31.08.2019 20:00

Mathematics, 31.08.2019 20:00

Mathematics, 31.08.2019 20:00

Mathematics, 31.08.2019 20:00

History, 31.08.2019 20:00

History, 31.08.2019 20:00











Mathematics, 31.08.2019 20:00

Mathematics, 31.08.2019 20:00

Business, 31.08.2019 20:00

Social Studies, 31.08.2019 20:00